Product Consultation
Your email address will not be published. Required fields are marked *


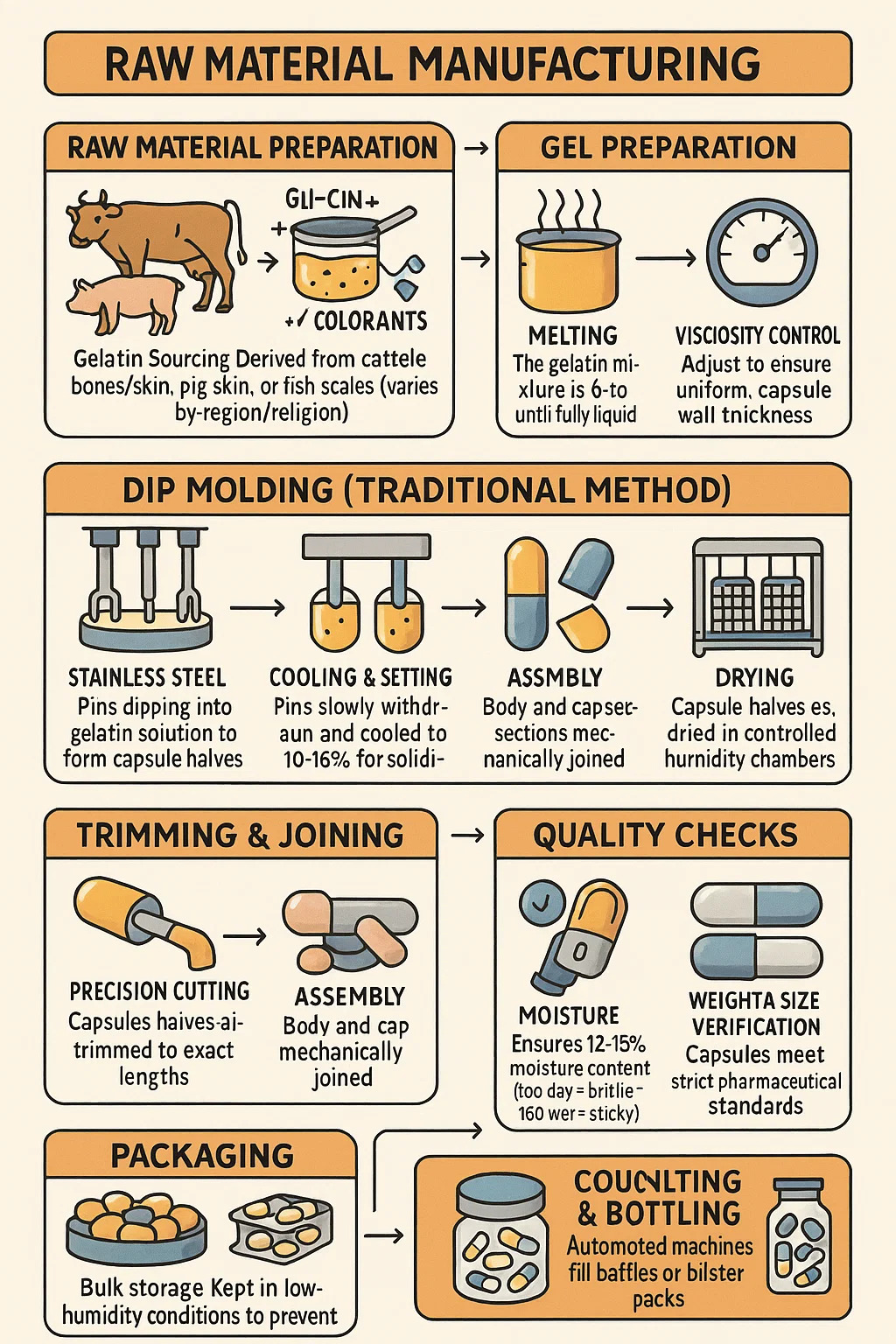
Gelatin Capsule Manufacturing Process
1. Raw Material Preparation
Gelatin Sourcing: Derived from cattle bones/skin, pig skin, or fish scales (varies by region/religion).
Purification: Boiled in acid/alkali solutions to remove fats and minerals, then filtered to remove impurities.
Blending: Mixed with water, glycerin (plasticizer), and sometimes colorants to form a thick gel.
2. Gel Preparation
Melting: The gelatin mixture is heated to 60-70°C until fully liquid.
Viscosity Control: Adjusted to ensure uniform capsule wall thickness.
Defoaming: Vacuum-treated to remove air bubbles that could weaken capsules.
3. Dip Molding (Traditional Method)
Stainless Steel Pins: Dipped into the gelatin solution to form capsule halves.
Cooling & Setting: Pins are slowly withdrawn and cooled to 10-15°C for solidification.
Drying: Capsule halves are dried in controlled humidity chambers to prevent warping.
4. Trimming & Joining
Precision Cutting: Capsule halves are trimmed to exact lengths.
Assembly: Body and cap sections are mechanically joined.
Polishing: Rolled in cloth to smooth edges and remove excess gelatin.
5. Quality Checks
Visual Inspection: Rejects deformed, discolored, or uneven capsules.
Moisture Testing: Ensures 12-15% moisture content (too dry = brittle; too wet = sticky).
Weight & Size Verification: Capsules must meet strict pharmaceutical standards.
6. Packaging
Bulk Storage: Kept in low-humidity conditions to prevent softening.
Counting & Bottling: Automated machines fill bottles or blister packs.
| Stage | Key Process Details | Critical Notes |
| 1. Raw Material Prep | • Gelatin extraction: Boiling cattle/pig/fish parts in acid/alkali baths | – Halal/Kosher: Requires certified animal slaughter |
| • Additives: Glycerin (plasticizer), purified water, optional dyes | – No alcohol-based solvents permitted | |
| 2. Gel Melting | • Heated to 60–70°C until liquid | – Viscosity control ensures uniform capsule walls |
| • Vacuum defoaming removes air bubbles | – Stainless steel tanks only (no reactive metals) | |
| 3. Dip Molding | • Stainless steel pins dipped into gel, cooled to 10–15°C for solidification | – Dedicated molds: No cross-contamination with non-halal lines |
| • Dried in humidity-controlled chambers (~30% RH) | – Slow drying prevents brittleness | |
| 4. Trimming/Joining | • Capsule halves cut to precise length, joined mechanically | – No animal-fat lubricants in machinery |
| • Polished with food-grade cloths | – Metal detectors check for equipment debris | |
| 5. Quality Control | • Visual inspection: Rejects deformed/discolored capsules | – Moisture testing: 12–15% target (critical for shelf life) |
| • Weight/size checks: Automated sorting | – Dissolution testing verifies drug release rates | |
| 6. Packaging | • Bottled or blister-packed in low-humidity environments | – Barrier packaging prevents moisture absorption |
Your email address will not be published. Required fields are marked *
If you would like to learn more about our products, please feel free to contact us and we will do our to assist you.
No.1 Tianzhu 3rd Road, Dufu Town, Xinchang County, Zhejiang Province
86-575 8606 0065
86-159 8825 2009
+86 159 8825 2009
+1 380 215 7432
